Agile Development Process - Embedded Systems
The Challenge
Establishing an agile DevOps process for embedded software development presents several unique challenges compared to traditional software development environments. Here are some key challenges:
Hardware dependencies: Embedded software development often involves tight integration with hardware components. Unlike traditional software, changes to embedded systems may require adjustments to physical components. Coordinating hardware changes with software iterations can be challenging and may require close collaboration between hardware and software teams.
Limited resources: Embedded systems typically have resource constraints such as limited memory, processing power, and energy consumption requirements. This restricts the use of certain tools and practices common in agile and DevOps processes. Teams must carefully manage resource usage to ensure that the software meets performance and efficiency requirements.
Testing complexities: Testing embedded software can be more challenging than testing traditional software due to the interaction between software and hardware. Automated testing may be limited or more difficult to implement, and comprehensive testing may require specialized equipment or environments. Additionally, testing on actual hardware may be necessary, which can slow down the development cycle.
Regulatory compliance: Many embedded systems are subject to regulatory requirements and industry standards, particularly in safety-critical applications such as automotive or medical devices. Compliance with these standards adds additional constraints and overhead to the development process, potentially complicating the adoption of agile and DevOps practices.
Long development cycles: Embedded software development often involves longer development cycles compared to other types of software. This can be due to factors such as hardware design cycles, rigorous testing requirements, and the need for firmware updates in the field. Agile and DevOps practices may need to be adapted to accommodate these longer cycles while still delivering value to customers incrementally.
Cross-disciplinary collaboration: Successful embedded software development requires collaboration between software engineers, hardware engineers, and other stakeholders such as product managers and quality assurance teams. Aligning these diverse teams around agile and DevOps practices may require cultural and organizational changes, as well as effective communication and coordination mechanisms.
Elco Solutions CT/CT Pipeline
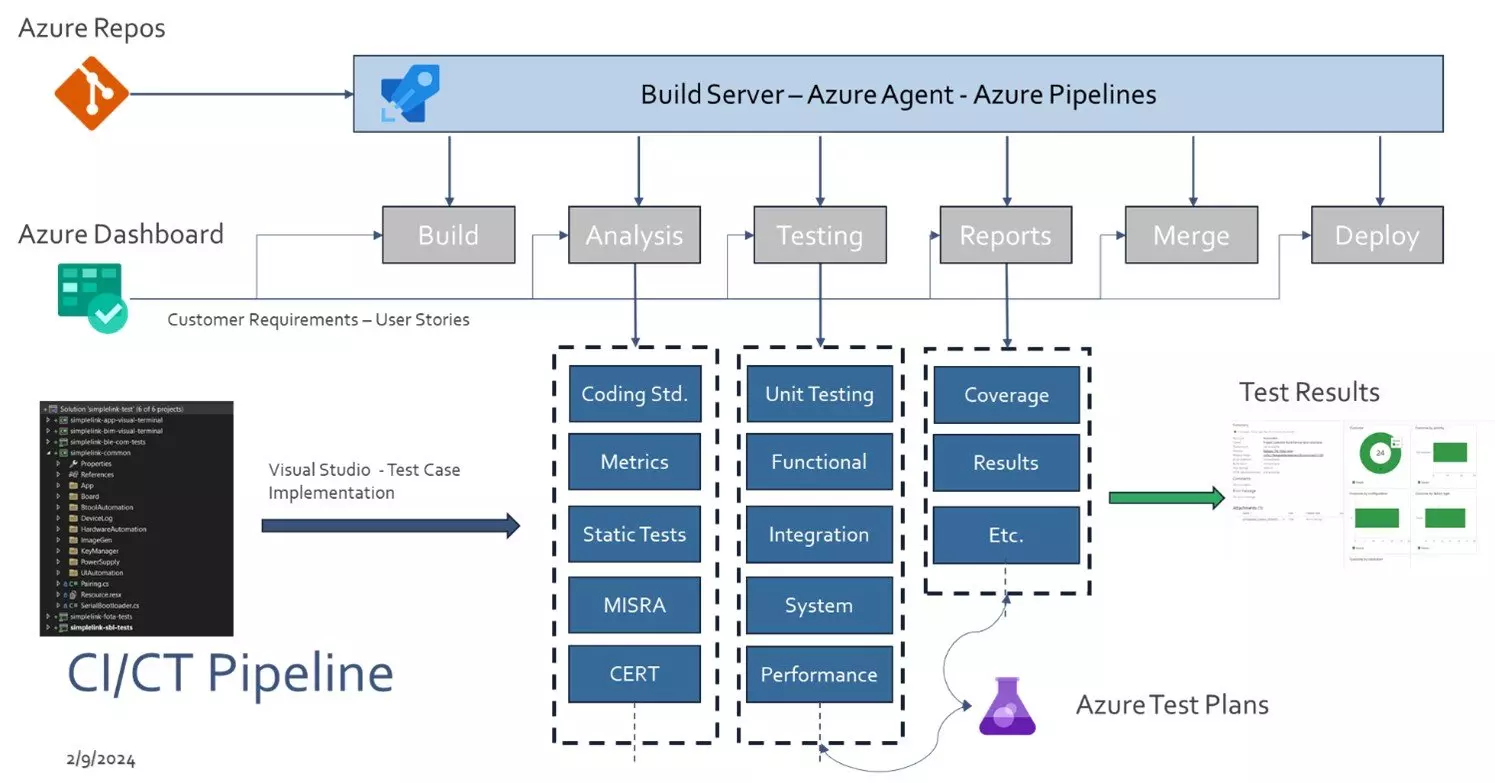
Addressing these challenges of DevOps for Embedded Systems requires a combination of technical expertise, process improvement, and organizational alignment. Adopting agile and DevOps practices in embedded software development can lead to faster time-to-market, improved product quality, and greater customer satisfaction, but it requires careful planning and adaptation to the unique characteristics of embedded systems development.
Elco Solutions has established a software development process that adheres to international standards that specifies lifecycle requirements for the development of high-quality software in the medical, automotive and factory automation industries.
This Case Study outlines the processes and activities necessary for the safe design, development, and maintenance of high-quality software including the requirements related to:
Risk Management: Identifying and mitigating potential risks associated with the software throughout its lifecycle.
Software Development: Establishing processes for software development, including requirements management, design, implementation, verification, and validation.
Configuration Management: Managing changes to the software, including version control and documentation.
Software Maintenance: Implementing procedures for maintaining and updating the software, including addressing defects and improvements.
Documentation: Generating and maintaining documentation to demonstrate compliance with regulatory requirements and standards.
Verification and Validation: Conducting activities to verify that the software meets its specified requirements and validate that it performs as intended in its intended environment.
Software Risk Classification: Classifying the software based on its potential impact on patient safety and regulatory requirements.


